Arterial blood gas analyzers have been available for laboratory and point-of-care (POC) use for decades. In 1957, John Severinghaus developed the first blood gas analyzer (now located in the Smithsonian), which measured pH, PCO2, and PO2. In the mid 1960s, blood gas analyzers became commercially available and were used primarily in clinical laboratories. As sensors, electronics, and fluidics improved, analyzers became smaller and easier to use, and gradually migrated into POC areas such as respiratory therapy and pulmonary function.
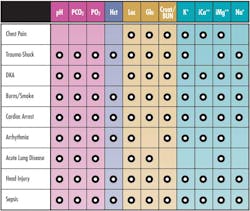
With the introduction of new whole blood biosensors, the role of analyzers expanded significantly. In 1985, the first combined blood gas/electrolyte analyzer was introduced, with a menu of pH, PCO2, PO2, Na, K, iCa, and hematocrit. As additional whole blood biosensors were introduced, blood gas analyzers evolved into comprehensive critical care analyzers, capable of performing a broad menu including pH, PCO2, PO2, Na, K, Cl, iCa, iMg, glucose, lactate, urea, creatinine, hematocrit, hemoglobin, O2 saturation, and co-oximetry. With dramatically expanded capabilities, these analyzers are now employed routinely in critical care areas such as ICU, NICU, ED, and surgery. Expanded test menus enable critical care blood gas analyzers to provide actionable results in many critical indications (Table 1).
Critical care blood gas analyzers
Each type of analyzer possesses different operational and economic characteristics:
- Bench-top critical care analyzers use individual biosensor technology that has been proven to be accurate and validated over many years. These analyzers typically have the broadest test menu and lowest cost of operation. Each biosensor can be individually replaced on a scheduled basis.
- Cartridge-based POC analyzers utilize all-in-one cartridges that were developed for easier use. Non-laboratory personnel such as respiratory therapists and nurses can maintain the analyzer by periodically replacing a single cartridge containing the sensors, calibrator solutions, and, in some cases, quality control (QC) solutions, all at once. However, while replacing a combined cartridge may minimize maintenance time, it could also be more costly, as unused reagents or remaining sensor life may be discarded. Additionally, a problem isolated to any one component could only be solved by replacing all components.
- Handheld, portable analyzers with single-use, disposable cartridges allow for testing at the bedside and in an ambulance. Though portable, these devices can incur higher operational costs than bench-top and POC critical care analyzers.
The next evolution of critical care blood gas analyzers—happening now—employs a POC approach using individual cartridges with all sensors miniaturized into one micro-sensor “card” and the calibrator and QC solutions contained in separate, individual, replaceable cartridges. Individual cartridge replacement optimizes the life of all components, greatly reducing cost and maintenance—issues that have been a challenge for cartridge-based analyzers and bench-top analyzers, respectively. Automatic, true liquid QC; continuous electronic self-monitoring; and self-verification of correct analyzer performance ensure testing quality and regulatory compliance.
2016 CMS requirements
CMS published new requirements under CLIA for laboratory QC that eliminate equivalent quality control (EQC) plans that have been used for some analyzer models. The new CLIA
regulations recognize that the use of EQC plans, which provide internal electronic monitoring, as the primary means to ensure the quality of analyzer results are flawed and do not provide an effective substitute for liquid-based QC. The new CLIA requirements call for daily liquid QC, or, as an alternative, an individualized quality control plan (IQCP) based on risk management:
- For blood gas analyzers, perform a minimum of three QC per day, or follow the manufacturer’s requirement for frequency of external quality controls, whichever is greater.
- Develop an IQCP for a test system that is based on principles outlined in CLSI document EP23, Laboratory Quality Control Based on Risk Management; Approved Guideline.
An IQCP takes into consideration the full risk profile of using an analyzer. The risk assessment identifies and evaluates potential failures and errors in the entire testing process, taking into consideration the risk of reporting a bad result. With high acuity, critically ill patients whom blood gas analyzers are expected to support, this risk profile is essential to developing a safe and effective IQCP.
CMS initiated a two-year educational/implementation period for the new quality control requirements that will end January 1, 2016. As this deadline approaches, providers that utilize critical care blood gas instrumentation will have to evaluate what “good laboratory practice” means for their critical care testing program.
Stringent QC/QA required
In developing a quality control plan, users must consider the vital role of critical care analyzers and the medical necessity for accuracy. Test results direct treatment in life-threatening situations. The risk of inaccurate POC results could be fatal, as has been the case in bedside glucose testing. The need for immediate, accurate results demands continuous, vigilant oversight of analyzer status—through QC checks. The routine use of liquid QC with known assay values is the only true way to verify the accuracy and proper functioning of critical care blood gas analyzers. Multi-level liquid QC verifies accurate performance throughout the full measurement range for all analytes.
Many of today’s critical care blood gas analyzers provide onboard QC that can be run automatically or on demand (e.g., to confirm a critical result). In addition, QC lockout features can prevent results reporting in the event of a QC failure. QC statistics verify the medical requirements for reproducibility within run and day to day. Continuous, internal self-monitoring of all analytical components provides an additional level of QA between QC runs. QC peer group programs with inter-laboratory performance data provide yet another level of QA. Regardless of which type of blood gas analyzer is employed and which CLIA option is chosen, these features should be the foundation of any comprehensive, QC program for critical care blood gas testing.
Brad Bullen, BS, RCP, serves as Sales Product Line
Manager—Core Products for Nova Biomedical.
Rick Rollins, MBA, serves as Associate Marketing Manager for Nova Biomedical.